Contact:
4008465777
Tonghua Dongbao releases 2023 sustainability report
On March 28, Tonghua Dongbao Pharmaceutical Co., Ltd. (stock code: 600867, hereinafter referred to as "Tonghua Dongbao" or the "Company") released its 2023 sustainability report. The report disclosed the Company's practices and achievements over the past year in areas including environmental protection, climate change response, R&D and innovation, product quality, and corporate governance.
Accelerated R&D and rigorous quality control
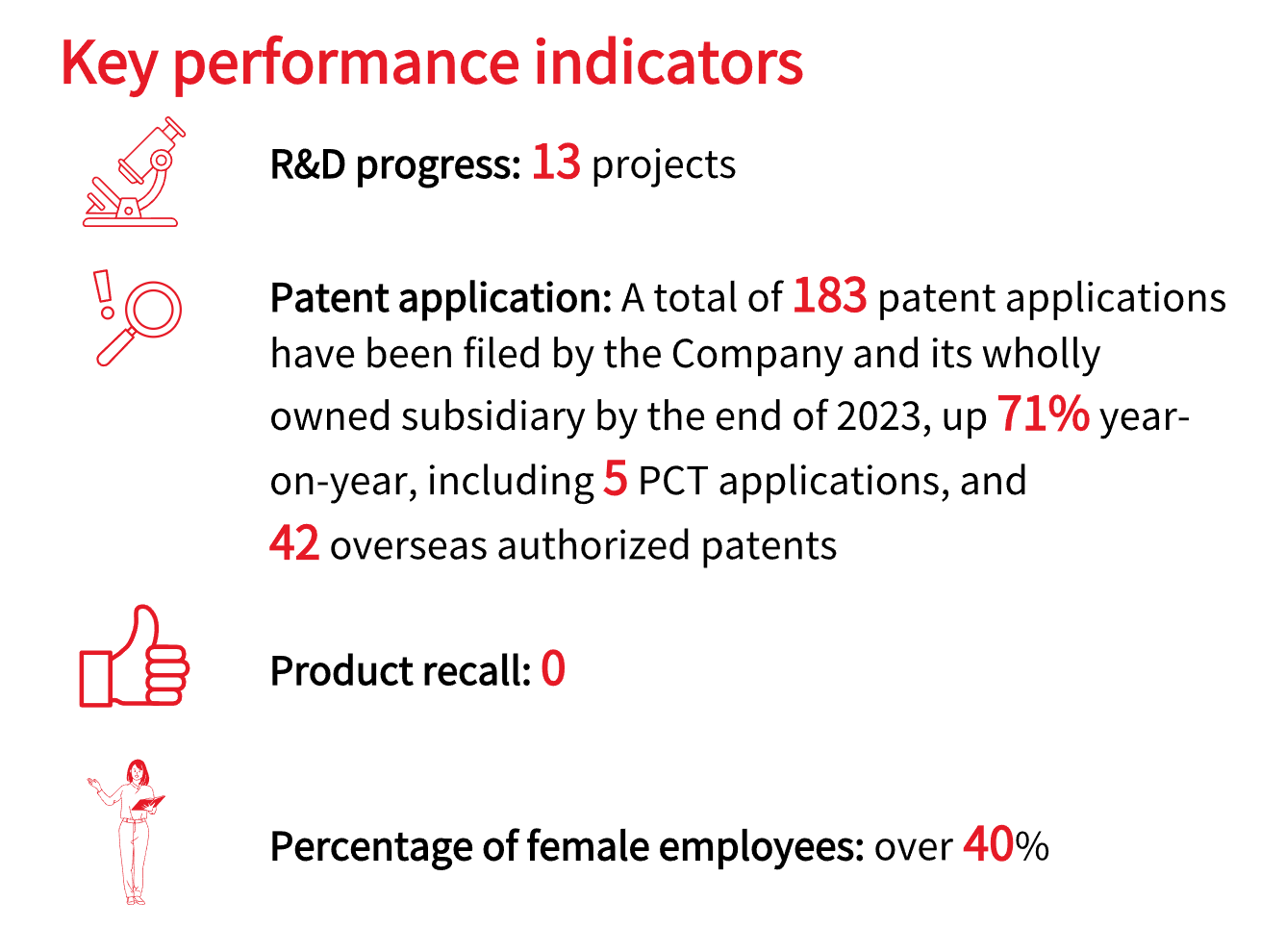
Tonghua Dongbao is dedicated to meeting the unmet needs of patients with endocrine and metabolic diseases. During the reporting period, the Company increased its R&D spending to RMB 420 million, accounting for 13.67% of its total revenue. Clinical trials for novel drugs progressed efficiently. Two Class 1 drugs, i.e., an oral small molecule GLP-1 receptor agonist (THDBH110 capsules) and a dual GLP-1/GIP receptor agonist (THDBH120 injection), have both completed the enrollment of the first subject in Phase I clinical trials. In addition, the NMPA has accepted the clinical trial application for THDBH120 injection intended for weight loss indications. A novel URAT1 inhibitor (THDBH130 tablets) achieved its primary endpoint in Phase IIa clinical trials; a potential first-in-class dual-target XO/URAT1 inhibitor (THDBH151 tablets) also met its primary endpoint in Phase I trials.
As of 2023, the Company and its wholly owned subsidiary Dongbao Zixing (Hangzhou) Biopharmaceutical Co., Ltd. had filed 183 patent applications, up about 71% year on year, including five PCT applications. These patents cover countries and regions such as Europe, the United States, Japan, Brazil, Mexico, and Indonesia. Currently, we have 98 issued patents, up more than 100% year on year, with 56 granted in China and 42 granted in other countries, compared to one in 2022.
Drug quality is the cornerstone of our business. We maintain strict control over all stages of drug production, implementing a comprehensive quality management system that covers material inspection, production control, drug release, and after-sales services. We ensure that all employees have a strong awareness of quality risk management. Our marketed products have obtained GMP certification from the National Medical Products Administration.
We have established an independent department that is responsible for improving pharmacovigilance and product recall systems to ensure drug safety for patients. During the reporting period, the Jilin Drug Safety Monitoring Center conducted a routine pharmacovigilance inspection of the Company and found no serious or major deficiencies.
Cash dividends for 13 consecutive years to protect investor rights and interests
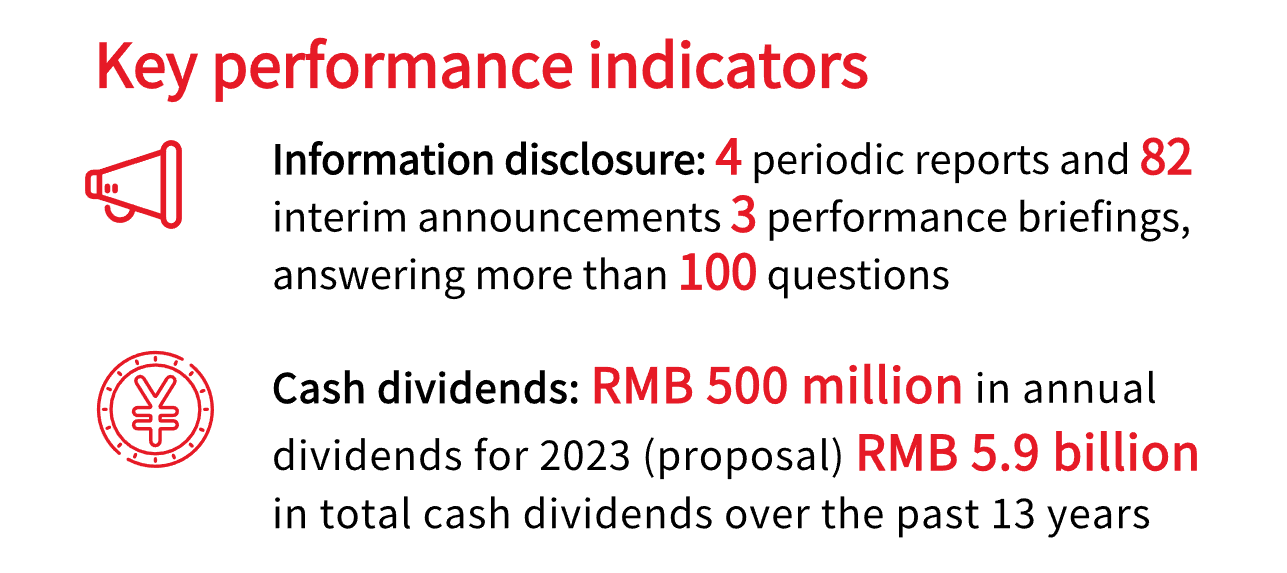
We care about the rights and interests of our shareholders. To enhance information disclosure and investor relations management, we promptly update our shareholders on significant matters and address their concerns. In 2023, the Company prepared and disclosed four routine reports and 82 interim announcements. We held three performance briefings during which we responded to over 100 questions from investors on the SSE E-interactive platform.
As our scale and profitability continue to grow, we remain committed to providing investors with fair returns, fully safeguarding the lawful rights and interests of our shareholders. We intend to pay a cash dividend of RMB 2.50 per 10 shares (tax included) for 2023, totaling about RMB 500 million. Since 2011, we have paid cash dividends for 13 years in a row. If the profit distribution proposal for 2023 is approved at the shareholders' meeting, the cumulative cash dividends issued will reach around RMB 5.9 billion, including share buybacks equivalent to cash dividends.
Climate action and carbon neutrality
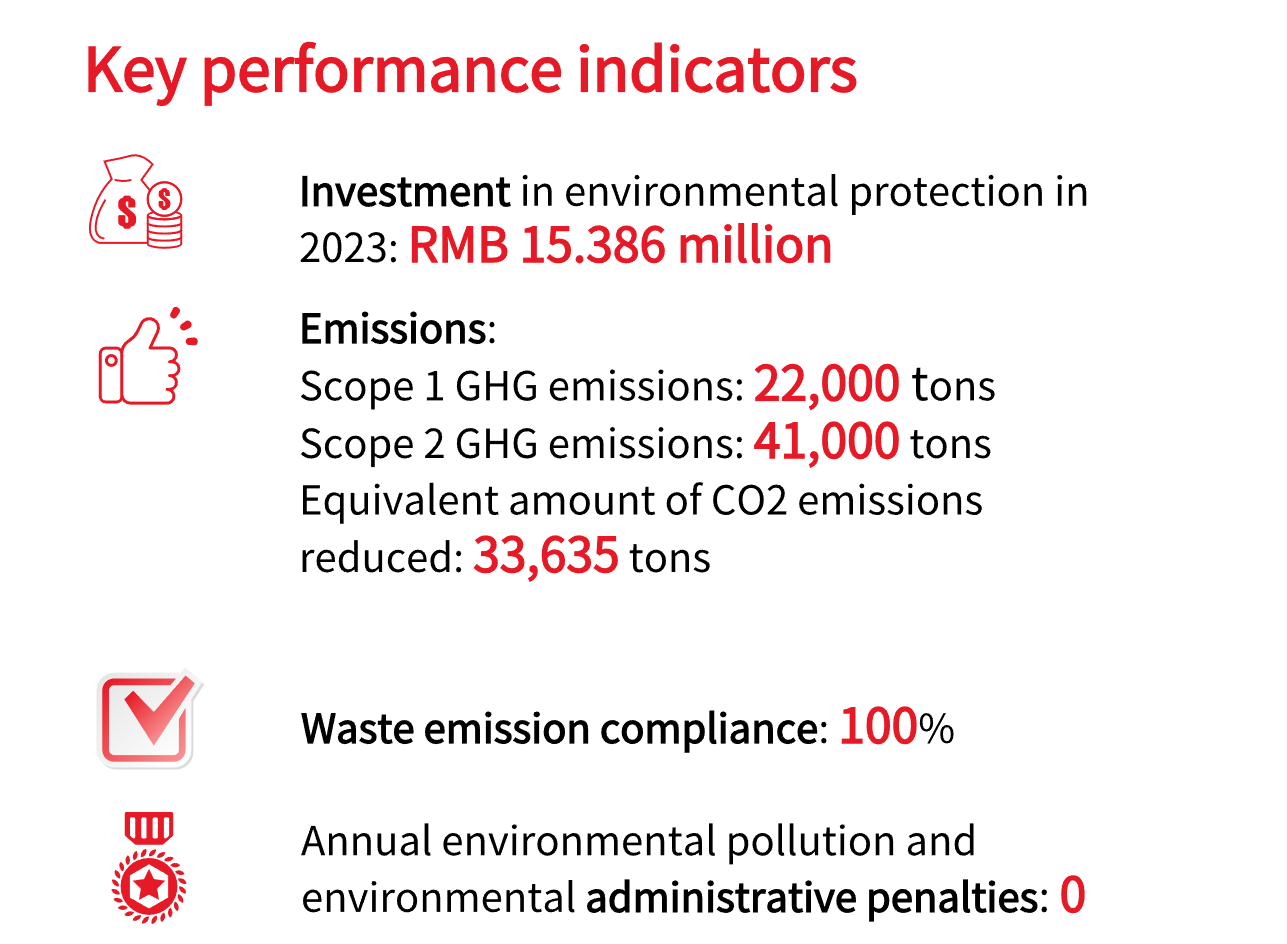
Climate change is becoming one of the biggest threats to humanity, affecting the ecosystem, environment, and many aspects of our economy and society. Following TCFD recommendations and employing scenario analysis, we have identified six key risks and opportunities in assessing the costs and resources involved in transitioning to a low-carbon business model. This helps us better understand the potential impacts of climate change on our day-to-day operations. Our boiler fuels have all transitioned from coal to natural gas, reducing our CO2 emissions by approximately 30,000 tons in 2023. Moreover, we disclosed our Scope 1 and Scope 2 greenhouse gas (GHG) emissions for the first time in 2023.
In 2024, we will upgrade our boiler flue gas heat recovery system, which is expected to reduce CO2 emissions by about 411 tons annually.
Moving forward, we will remain committed to fulfilling our obligations towards all stakeholders. We aim to align our business operations with social responsibility, striving for sustainable, high-quality development in economic, social, and environmental dimensions.










